siRNA Drug Development Program
Bioneer's siRNA Drug Development Program using SAMiRNA Technology
Vertically integrated processes within Bioneer's siRNA Drug Development Program provide a total solution for siRNA therapeutics discovery and development, from siRNA design/synthesis and preclinical tests to IND filing. With its world's-best RNAi core technologies and manufacturing infrastructure, Bioneer is the ideal partner for pharmaceutical and biotechnology companies currently working on the development of RNAi therapeutics, or seeking to enter the RNAi field. Bioneer's research and technical support teams ensure top-quality products and services to meet your unique needs.
Our siRNA Drug Development Program includes:
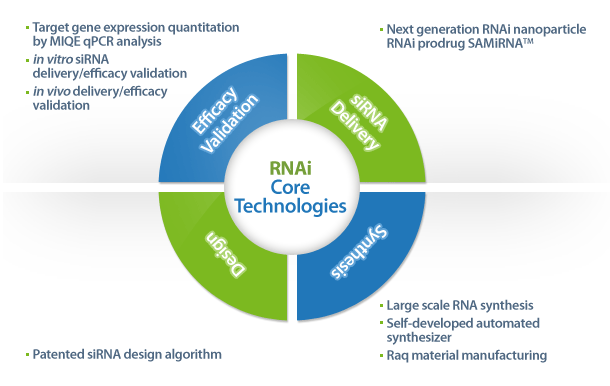
- Design and synthesis of siRNAs (custom siRNA synthesis using patented design algorithm, which has been used for genome-wide siRNA libraries for human, mouse and rat)
- in vitro screening and validation of siRNAs including MIQE qPCR analysis of knockdown rates
- Preclinical test using SAMiRNA including in vivo siRNA delivery and biodistribution/efficacy test using murine and non-human primate models
- Large scale manufacturing of SAMiRNA (in house raw material manufacturing facilities, fully automated siRNA/SAMiRNA synthesis pipeline)
- Drug candidates efficacy/toxicity tests in nonhuman primate (through collaboration with Korea Institute of Toxicology)

Design and Synthesize siRNAs
Genome-wide siRNA libraries with guaranteed KD: 132,000 predesigned siRNAs for 44,000 target genes from Human, Mouse and Rat
Custom siRNA synthesis using patented Turbo siRNA design algorithm With our nucleic acid manufacturing and development expertise, Bioneer has been providing top-quality DNA oligos and siRNAs worldwide for close to 20 years
In vitro screening and validataion of siRNAs
- In-house mammalian cell culture facilities
- Large scale high-throughput in vitro screening work flow established: capability of HTP Validation of siRNA
- The entire R&D Process is vertically integrated – including the exclusive use of Bioneer reagents – for unparalleled quality control every step of the way
MIQE qPCR analysis
- Validation of target gene knockdown and off-target effects is a key step for "best-in-class" siRNA discovery
- qPCR gene expression profiling service procedure under MIQE guidelines provides accurate and robust data generation
- Transcriptome analysis through Deep Sequencing (NGS) for off-target effect analysis.
Preclinical in vivo test using SAMiRNA
SAMiRNA: Nanoparticles targeting disease RNA
- Nanoparticle prodrug technology
- Strong IP position around SAMiRNA™'s unique structure & manufacturing processes
- In vivo efficacy validated in animal disease models
- PK/PD tests for serum stability
- Toxicity and cytokine induction tests performed
- Fully automated solid phase chemical synthesis of siRNA conjugates
- Powerful siRNA delivery platform technology
Large scale manufacturing
BIONEER Developes & Mass-Produces siRNA
- In-house Raw material manufacturing plant
- Fully automated synthesis facility at Bioneer With a capacity of >30,000 siRNAs per day
- Clean Room Synthesis of Oligonucleotides & siRNA
Non human primate test, toxicity screen
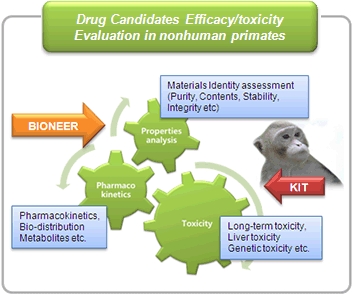
- MOU collaboration agreement between BIONEER and Korea Institute of Toxicology (KIT): Using KIT's expertise and experience on antisense oligo and miRNA drug toxicology evaluation (CRO for ISIS and Regulus for non-human primate tests)
- Fast track on entering IND filing and clinical trials




