Platform Technologies
㈜바이오니아가 개발한 고효율의 생분해성 자가조립 나노입자인 SAMiRNA™는 효율은 극대화되고 부작용은 최소화된 세계 최고 수준의 siRNA 핵심 신약기반 기술입니다. 당사의 SAMiRNA™ 기반 기술을 활용하면 다양한 난치성 질환들이나 항암 target에 대한 치료제 개발에 활용 가능한 신약개발 후보를 만들 수 있습니다.
㈜바이오니아는 차세대 siRNA 치료제 플랫폼기술인 SAMiRNA™을 이용 기존의 항체, 화학합성 의약품으로 치료가 어려웠던 난치병 치료제의 개발을 목표로 연구 및 개발 역량을 집중하고 있습니다.
이의 일환으로 ㈜바이오니아는 현재 신약 후보물질을 보유한 국내외 생명공학회사들과의 공동 연구개발을 통해 제품 Pipeline을 구축하고 있으며, 글로벌 제약회사들과 전세계적인 블록버스터 신약 공동 개발을 목표로 하고 있습니다.
SAMiRNA™ (Self-Assembled-Micelle-inhibitory-RNA)
SAMiRNA™는 세계 최초로 개발된 siRNA 나노입자 기반 prodrug 입니다. 생 분해성의 자가조립미셸 siRNA 나노입자 (SAMiRNA, Self-Assembled Micelle siRNA Nanoparticle)로 뛰어난 전달효과 및 siRNA 약효를 나타냅니다. 암과 다양한 질병모델 치료제로 적용 가능합니다.
특허 출원된 독창적인 구조의 자가조립 siRNA 나노입자 (Fig 1)는 외부의 PEG 층에 의해 내부 siRNA 가 보호되는 형태를 가집니다. 최적화된 나노 스케일의 사이즈로 EPR (Enhanced Permeability and Retention)효과에 의해 종양조직이나 SAMiRNA 표면에 부착된 타겟팅 물질에 의해 목표 질병 장기로 선택적으로 전달됩니다.
Key features of SAMiRNA™
: Best-in-class RNAi drug SAMiRNA overcomes the Unmet needs in siRNA drug development
| Unmet needs in siRNA drug development | SAMiRNA™ |
| Adverse effects | No detectable liver toxicity or innate immune response |
| Delivery (Issues with other technologies: rapid clearance and degradation in blood, No target tissue specificity, low in vivo efficacy) | Outstanding serum stability (PK/PD validated), tumor/tissue targeting capabilities verified, long lasting in vivo efficacy validated in animal disease models |
| Manufacturing cost | One-step automated solid-phase synthesis of SAMiRNA and rapid chromatographic purification process: enormous advantage allowing for economical large scale production of SAMiRNA. |
| QC processes | Since SAMiRNA is a Single Chemical Entity the QC process is dramatically simplified |
| Expansion of indications | Powerful siRNA delivery platform technology with flexibility to incorporate siRNA (or miRNA or antisense) sequences against any disease target; Your target gene(s) can be easily transformed into SAMiRNA nanoparticle! |
SAMiRNA™ Summary
Best-in-class RNAi prodrug technology: SAMiRNA™ is the most unique and effective RNAi prodrug developed to date for the treatment of cancer and other diseases.

Pre-clinical data for SAMiRNA
 Novel self-Assembling siRNA nanoparticles with a protective PEG coat and optimal monodisperse nano-scale size(Figure 1)
Novel self-Assembling siRNA nanoparticles with a protective PEG coat and optimal monodisperse nano-scale size(Figure 1) siRNA Prodrug: highly stable in circulation with siRNA release and activity only after metabolism within target cells
siRNA Prodrug: highly stable in circulation with siRNA release and activity only after metabolism within target cells Outstanding serum stability (Figure 4)
Outstanding serum stability (Figure 4) in vivo efficacy validated in animal disease models: completion of preclinical tests for cancer treatment (Figure 2)
in vivo efficacy validated in animal disease models: completion of preclinical tests for cancer treatment (Figure 2) Extremely low toxicity and cytokine induction (Figure 5-7)
Extremely low toxicity and cytokine induction (Figure 5-7) Combined with various targeting moieties, SAMiRNA nanoparticles can target various organs of interest, for example, liver
Combined with various targeting moieties, SAMiRNA nanoparticles can target various organs of interest, for example, liver
A new class of single molecule-based self-assembling Nanoparticles

Figure 1. Structure of SAMiRNA Nanoparticle.
(A) Schematic diagram of SAMiRNA
(B) Cryo-TEM images of SAM-siRNA Nanoparticles (scale bar = 100 nm).
Long-lasting in vivo efficacy validated in mouse cancer models
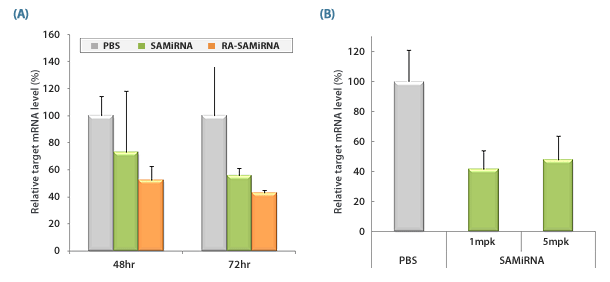
Figure 2. in vivo silencing of target mRNA by SAMiRNA Nanoparticels.
(A) Tumor bearing mice were intravenously injected with saline (PBS), or with a single 5mg/kg dose of either survivin-SAMiRNA, or tumor-targeting folic acid (FA)-conjugated survivin-SAMiRNA.
Mice were sacrificed at denoted time points and survivin mRNA levels in isolated tumor cell masses were subsequently measured using Real-Time PCR.
(B) Tumor bearing mice were intravenously injected with saline (PBS) and survivin-SAMiRNA NP at a single 1 or 5mg/kg dose.
Mice were sacrificed at 72 hr postinjection and survivin mRNA levels in isolated tumor cell mass were subsequently measured using real-Time PCR.
Tumor-specific targeting of SAMiRNA in mouse cancer models
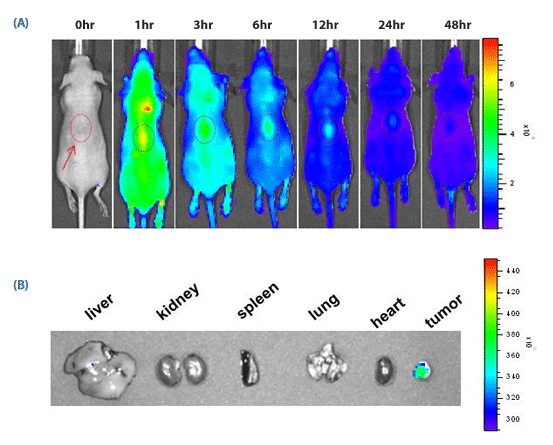
Figure 3. in vivo targeting of SAMiRNA Nanoparticles to s.c. grafted tumor.
(A) Time-dependent in vivo tumor targeting specificity of Cy5.5-labeled SAMiRNA, delivered via i.v. to tumor-bearing nude mice.
(B) Images of various organs extracted from treated mice 12 hours after injection. No fluorescence was detectable outside of the tumor proper.
in vivo PK/PD Quantification of i.v. administrated SAMiRNA™
Shows significantly enhanced stability compared to modified siRNA (2'-O-Methyl siRNA)
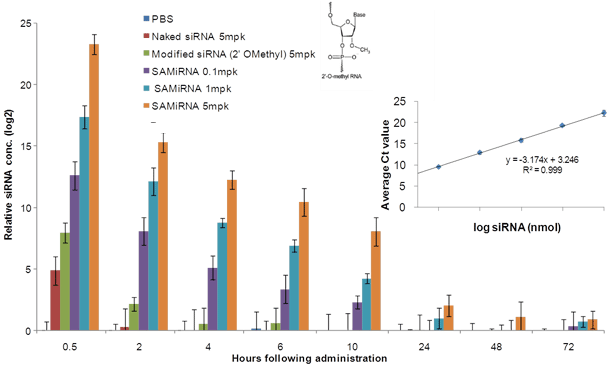
Figure 4. Quantification of siRNA in plasma from mice i.v. injected with SAMiRNA.
Mouse blood was extracted at indicated time points (hours post-injection) and SAMiRNA and siRNA levels in isolated plasma were measured by qRT-PCR assay.
The linear regression of the amplification curve shows an excellent R2 value for this assay.
SAMiRNA™ has no detectable adverse effects
Did not trigger any IFN response or liver toxicity
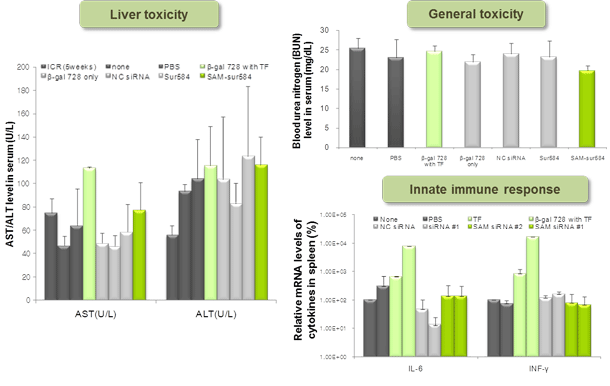
Figure 5. General toxicity test of SAMiRNA
Naked siRNA and SAMiRNA were i.v. injected into ICR mice in order to evaluate toxicity. Serum was collected 6 or 24 hr post-injection and toxicity markers were analyzed.
None (not-injected), ICR 5 weeks (average value from normal 5-week old ICR mice, from published literature), b-gal 728 (beta-galactosidase siRNA),
b-gal 728 with TF (beta-galactosidase siRNA mixed with in vivo MegaFectine from QBiogene), NC siRNA (negative control siRNA), Sur584 (survivin siRNA), SAM-sur584 (survivin siRNA-containing SAMiRNA).
SAMiRNA™ has no detectable adverse effects
Negligible cytokine induction in human PBMCs by SAMiRNA
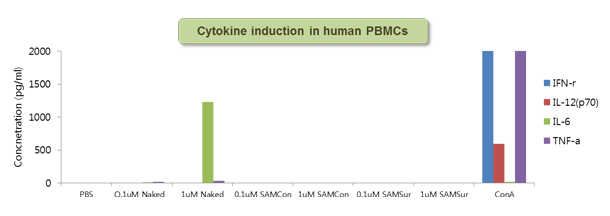
Figure 6. Cytokine response to SAMiRNA in human PBMC
Human whole blood was incubated with 0.1 or 1 uM of naked siRNA or SAMiRNA for 24 hrs at 37°C and the resulting supernatant was measured for various cytokine induction.
Concanavalin A (ConA) was used as positive control.
SAMiRNA™ has no detectable adverse effects
Extremely low toxicity profile of SAMiRNA
Independent results from a GLP-certified CRO, Biotoxtech Co. Ltd)
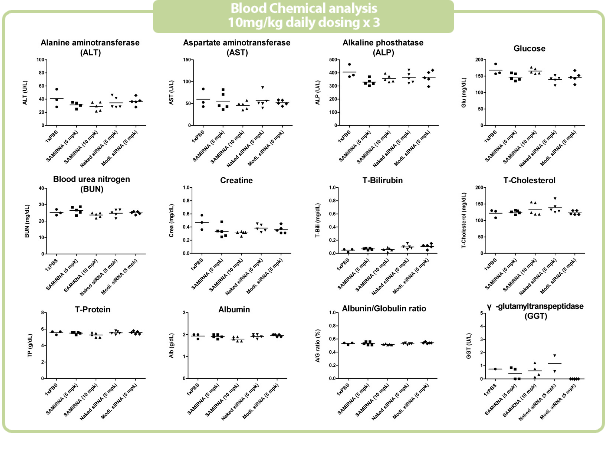
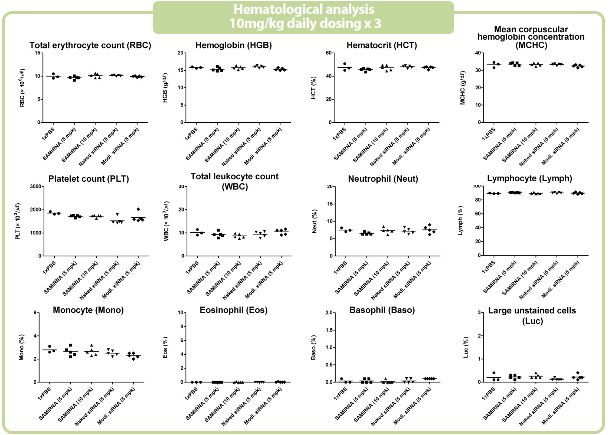
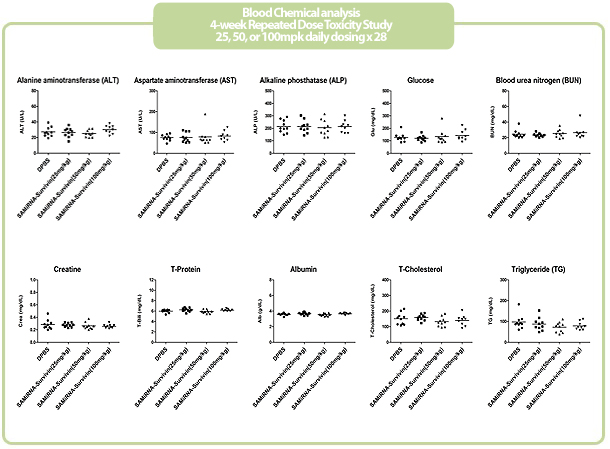
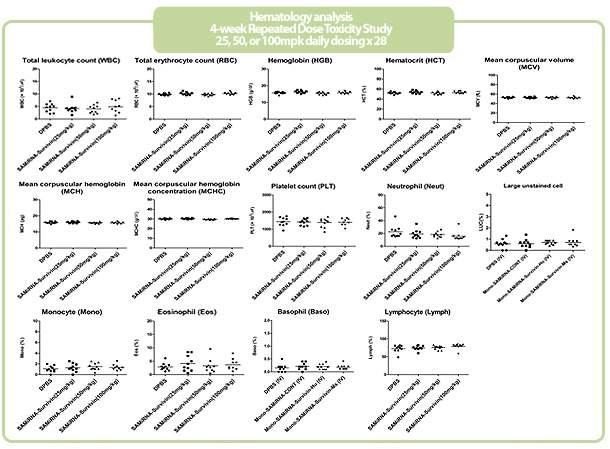
Figure 7. in vivo SAMiRNA blood chemical and hematological analysis
10 mpk of SAMiRNA, naked or modified siRNA was repeatedly i.v. injected to ICR mice (n=5) 3 times at intervals of one day.
Whole blood and separated serum were collected and analyzed 24 hrs after the final injection.
Toxicity test of SAMiRNA-Survivin including 28-day repeated dose
1. General Toxicology Study
1) Acute toxicity (100mpk, single dose)- Body weight changes
- Pathology findings
2) Repeated dose 28-Day (25, 50, 100mpk, multiple dose)
- Body weight changes
- Hematology parameters / Clinical chemistry parameters
- Urinalysis parameters
- Necropsy findings / Organ weighed / Tissue preserved
- Histopathological findings
2. Genetic Toxicology Study
1) Ames Test (5,000 ug per plate)- Signs of toxicity
- Individual plate counts
- Dose-response relationship
- Statistical analyses
- Concurrent negative (solvent/vehicle) and positive control data, with ranges, means and standard deviations
- Historical negative (solvent/vehicle) and positive control data, with e.g. ranges, means and standard deviations
2) Chromosome Aberration Assay (5,000 ug per plate)
- Cytotoxicity measurements
- Signs of precipitation
- Data on pH and osmolality of the treatment medium
- Definition for aberrations, including gaps
- Changes in ploidy
- Concentration-response relationship
- Statistical analyses
- Concurrent negative (solvent/vehicle) and positive control data
- Historical negative (solvent/vehicle) and positive control data, with ranges, means and standard deviations
Safety of SAMiRNA: 4-week repeated dose tox profiling
 Results
Results
1. General Toxicology Study
1) Acute toxicity- No acute toxicity including bodyweight changes and histological abnormalities were observed
2) Repeated dose 28-Day
- No clinically significant or dose-dependent changes were observed post-treatment in hematology, chemistry, urinalysis, coagulation parameters, reticulocyte counts, complement levels
- NOAEL (No Observed Adverse Effect Level) of SAMiRNATM was 100mg/kg/day
2. Genetic Toxicology Study
1) Ames test- No significant induction for mutagenic activity was observed with highest dose tested, 5,000ug per plate
2) Chromosome aberration assay
- No significant aberrations in chromosomes were noticed with highest dose tested, 5,000ug per plate
 Safety Assessment
Safety Assessment
Safety evaluation through 28-day repeated dose and genetic tox studies demonstrated that SAMiRNA is an exceptionally safe and efficient drug candidate
Safety of SAMiRNA: No adverse effects
Extremely low toxicity profile of SAMiRNA (by a GLP-certified CRO, Korea Institute of Toxicology)